UDI Unique Device Identifier Compliance
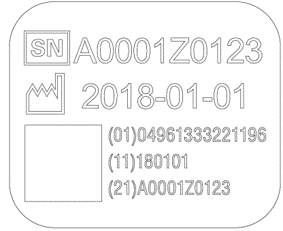
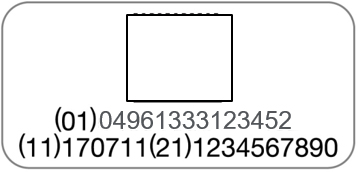
PENTAX Medical is adopting a UDI (Unique Device Identification) code required by Unique Device Identification System designed to adequately identify devices through distribution and use. The following information is coded in UDI symbol.
-(01) GS1 Commodity code (Global Trade Item Number)
-(11) Production date
-(21) Serial number
| Endoscope | Processor |
 |  |
If you have any questions, please contact your local PENTAX Medical representative.
Reference:
